how to find percent abundance
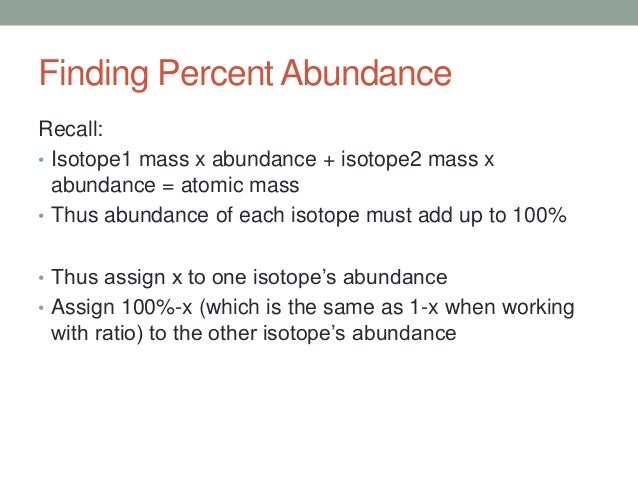
The formula to find the percent abundance of an element with two isotopes is as follows. The relative atomic mass is the weighted average of the isotopic masses.
C Isotopes Mass Atomic Ppt Video Online Download

Hw Study For Wednesday S Ch 8 Exam A 1 0 Mol Zinc B 1 0 Mol

Solution Boron Has Two Naturally Occurri Chemistry
To calculate the percent abundance of each isotope in a sample of an element chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.

How to find percent abundance of isotopes.
Abundance of 11 5 b 1000 abundance of 10 5 b.
Subtract this value from 100 percent to find the abundance of the other isotope.
Average mass of an element atomic mass of isotope i x percent abundance of isotope i100 atomic mass of isotope ii x percent abundance of isotope ii100.
How to calculate the percent abundance of an isotope identify atomic weights.
Set abundance equal to x.
Oxygen is composed of three isotopes.
Of the other two one has a mass of 15995 amu and the other has a mass of 17999 amu.
For example 100 1988 8012 percent.
Let x equal the percentage abundance of one of the two isotopes.
This is the percent abundance of b 11.
W 1 x w 2 1 x w e since the weights of both isotopes must add to give the weight of the element.
If you denote the abundance of isotope 1 by x the equation is.
This isotope makes up 0037 of oxygen.
Identify the elements atomic weight and the atomic count.
The sum of the percentages of the specific isotopes must add up to 100.
Since boron only has two isotopes the abundance of one must be 1000 the abundance of the other.
If youre given the mass of each isotope of an element and the average atomic mass you can calculate the percent abundance of each isotope.
Most elements are made up of a mixture of isotopes.
One has a a mass of 16999 amu.
The relative abundance of an isotope means the percentage of that particular isotope that occurs in nature.
Write out the equation for the elements atomic.
The percentages of multiple isotopes must add up to 100.
Calculate the abundance of the other two isotopes using the average atomic mass of 159994 amu.
/GettyImages-176643185-5899fe8a5f9b5874ee66ea0a.jpg)
How To Calculate Atomic Abundance From Atomic Mass

Answer An Element Has Three Isotopes Giv Clutch Prep

Helium Definition Properties Uses Facts Britannica

Abundance Of Isotopes
Atomic Theory And Structure

Isotopes If You Change The Number Of It Will Affect The And The

Calculating Percent Abundance Rubidium Rb Isotopes Example Youtube
Grade 9 U1 L11 Isotopes

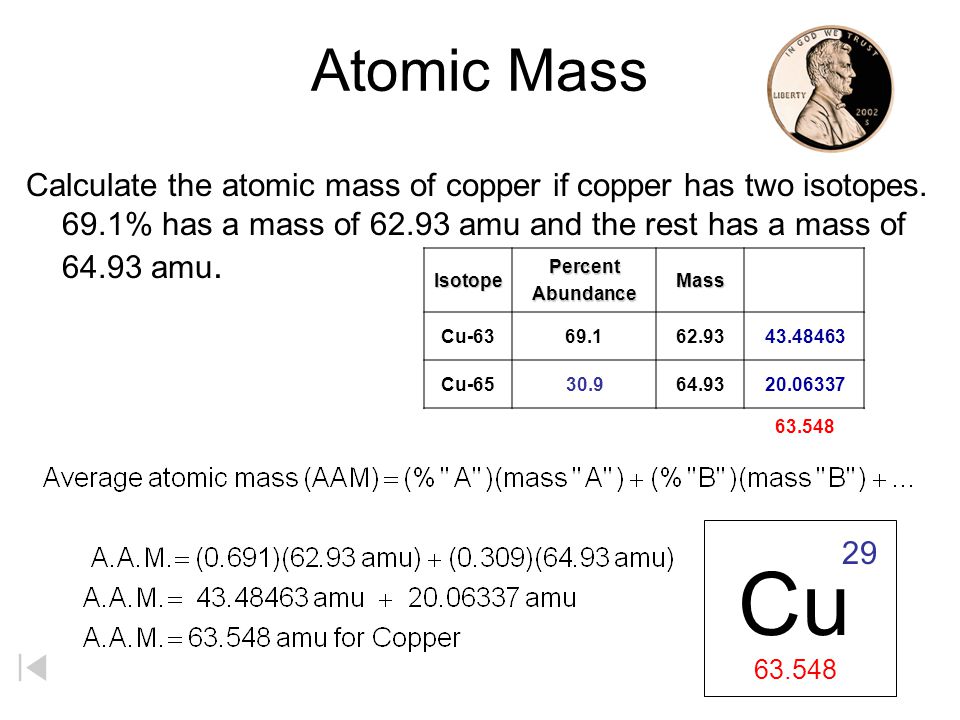
Atomic Mass

How To Calculate The Percent Abundance Of An Isotope

How To Find Average Atomic Mass 8 Steps With Pictures Wikihow

Finding Abundances For Two Isotopes Youtube

Calculating The Average Atomic Mass Steps For Calculating Average

Natural Abundance Of Isotopes Youtube

Isotope Composition Example Youtube
Https Www Foresthillshs Org Ourpages Auto 2017 9 25 60127506 Avg Atomic Mass Tc Pdf

Chemistry Practice Problems Atomic Mass Calculations Ii Get

Isotopic Composition Of Separated Nickel Isotopes Used In Ca
how to find percent abundance
Source: https://topptutors.blogspot.com/2014/12/how-to-find-percent-abundance-of.html
Posted by: simmonsvenswithe.blogspot.com

0 Response to "how to find percent abundance"
Post a Comment